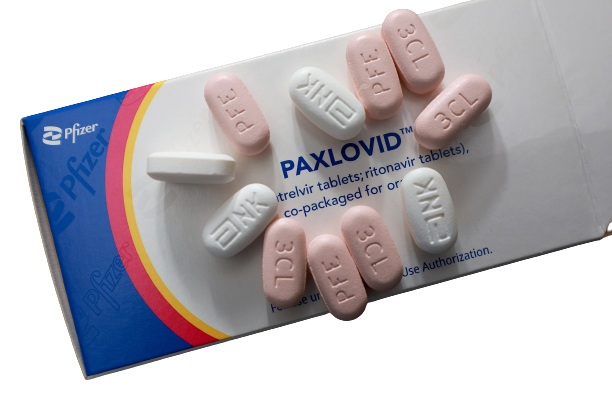
How is Paxlovid Used?
Paxlovid is provided in pill form, and doses are taken orally. One dose consists of two nirmatrelvir tablets and one ritonavir tablet, co-administrated twice daily for 5 consecutive days.
Antiviral medicines are most effective when taken as early as possible in the course of an illness. Paxlovid MUST be taken within five days of symptom onset.
- Take your second dose approximately 12 hours after. It is recommended you schedule your daily doses so they are easy to remember, for example, morning and evening.
- Take all three tablets (2 nirmatrelvir & 1 ritonavir) at the same time, or in immediate succession.
- Tablets should be taken whole – not chewed, broken or crushed.
- Be sure to complete the entire 5-day Paxlovid treatment regimen. Do not stop taking Paxlovid if your symptoms subside before the prescription has been finished as this can cause viral rebound and resistance to Paxlovid.
- Consult with your healthcare provider immediately if any issues arise.