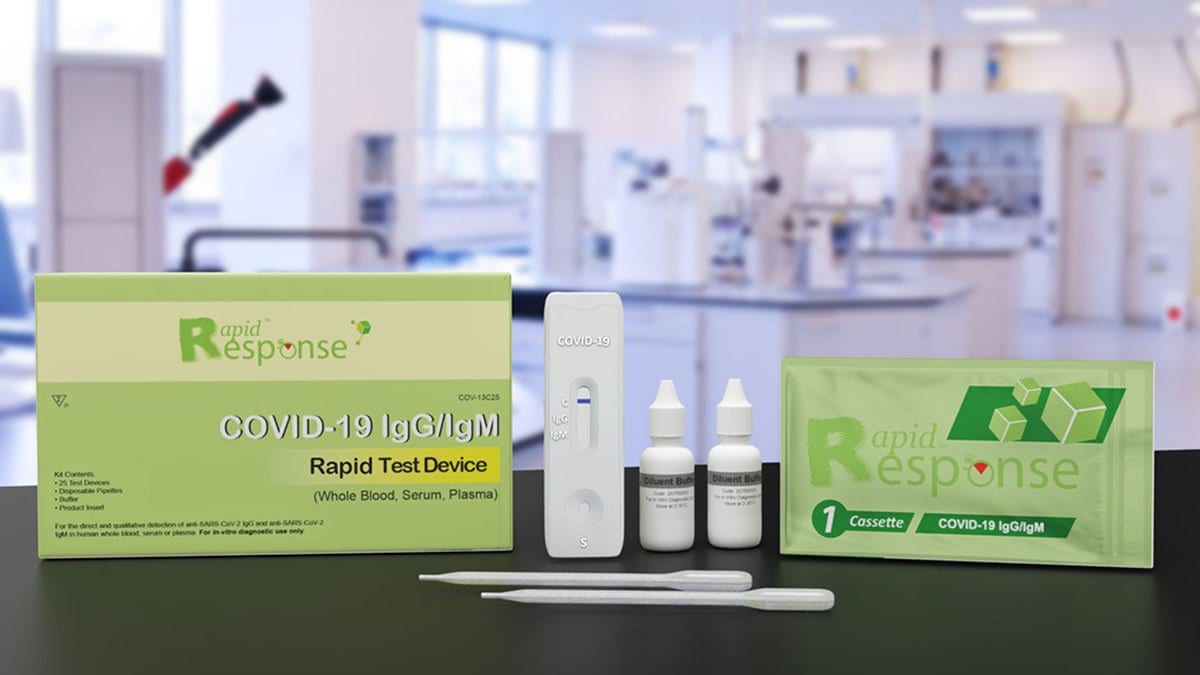
Product Code: COV-13C25
Sample: Whole Blood / Serum / Plasma
Format: Cassette
Quantity: 5 Tests/Box
Buffer solution: 1 bottle
Time to result: 15 minutes
Storage Condition: 2-30°C/35.6-86°F
Test Principle: Immunochromatographic Lateral Flow Assay
The Rapid Response™ COVID-19 IgG/IgM Rapid Test Device is an in vitro immunoassay for the direct and qualitative detection of anti-SARS-CoV-2 IgM and anti-SARS-CoV-2 IgG in human whole blood (including venous whole blood and capillary whole blood), serum, or plasma.
At the Point of Care setting, this test is only authorized for use with fingerstick whole blood specimens.
This test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. The Rapid Response™ COVID-19 IgG/IgM Rapid Test Device should not be used for screening patients or to diagnose or exclude acute SARS-CoV-2 infection.
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. A negative or non-reactive result for an individual subject indicates the absence of detectable COVID-19 virus antibodies. However, a negative or non-reactive test result does not preclude the possibility of exposure to or infection with the COVID-19 virus.
False-positive results may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false-positive results, confirmation of positive results should be confirmed using a second, different IgG/IgM detection method. Laboratories are required to report all results to the appropriate public health authorities. The test is for professional use only. This assay is not intended for home testing (or self-testing).
The Rapid Response™ COVID-19 IgG/IgM Rapid Test Device may detect a response to vaccination.
Pursuant to section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on March 18, 2020, the Rapid Response™ COVID-19 IgG/IgM Rapid Test Device is now authorized for sale or importation in Canada.
Clinical Evaluation Data
Clinical evaluation data for venous whole blood and serum samples:
- IgG Detection: Relative Sensitivity 94.6% (CI: 84.2%-98.6%), Relative Specificity: 100% (CI: 96.5%-100%)
- IgM Detection: Relative Sensitivity 92.9% (CI: 81.9%-97.6%), Relative Specificity: 98.1% (CI: 93.3%-99.5%
Intended Use
The BTNX COVID-19 IgG/IgM Rapid Test Device is a rapid lateral flow chromatographic immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood (sodium EDTA), serum, plasma (sodium EDTA) and fingerstick whole blood. The COVID-19 IgG/IgM Rapid Test Device is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The COVID-19 IgG/IgM Rapid Test Device
should not be used to diagnose acute SARS-CoV-2 infection.
Use of this test with all authorized specimen types is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform moderate or high complexity tests.
This test is also authorized for use with fingerstick whole blood specimens only at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Results are for the detection of SARS-CoV-2 antibodies. The IgG and IgM antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although the duration of time antibodies are present post infection is not well characterized. Individuals may have detectable virus present for several weeks following seroconversion.
Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
The sensitivity of COVID-19 IgG/IgM Rapid Test Device early after infection is unknown. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
False positive results for COVID-19 IgG/IgM Rapid Test Device may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay.
The COVID-19 IgG/IgM Rapid Test Device is only for use under the Food and Drug Administration’s Emergency Use Authorization.
By purchasing this product, you agree to follow the manufacturer’s intended use and the sale conditions of this website.
















































Reviews
There are no reviews yet.