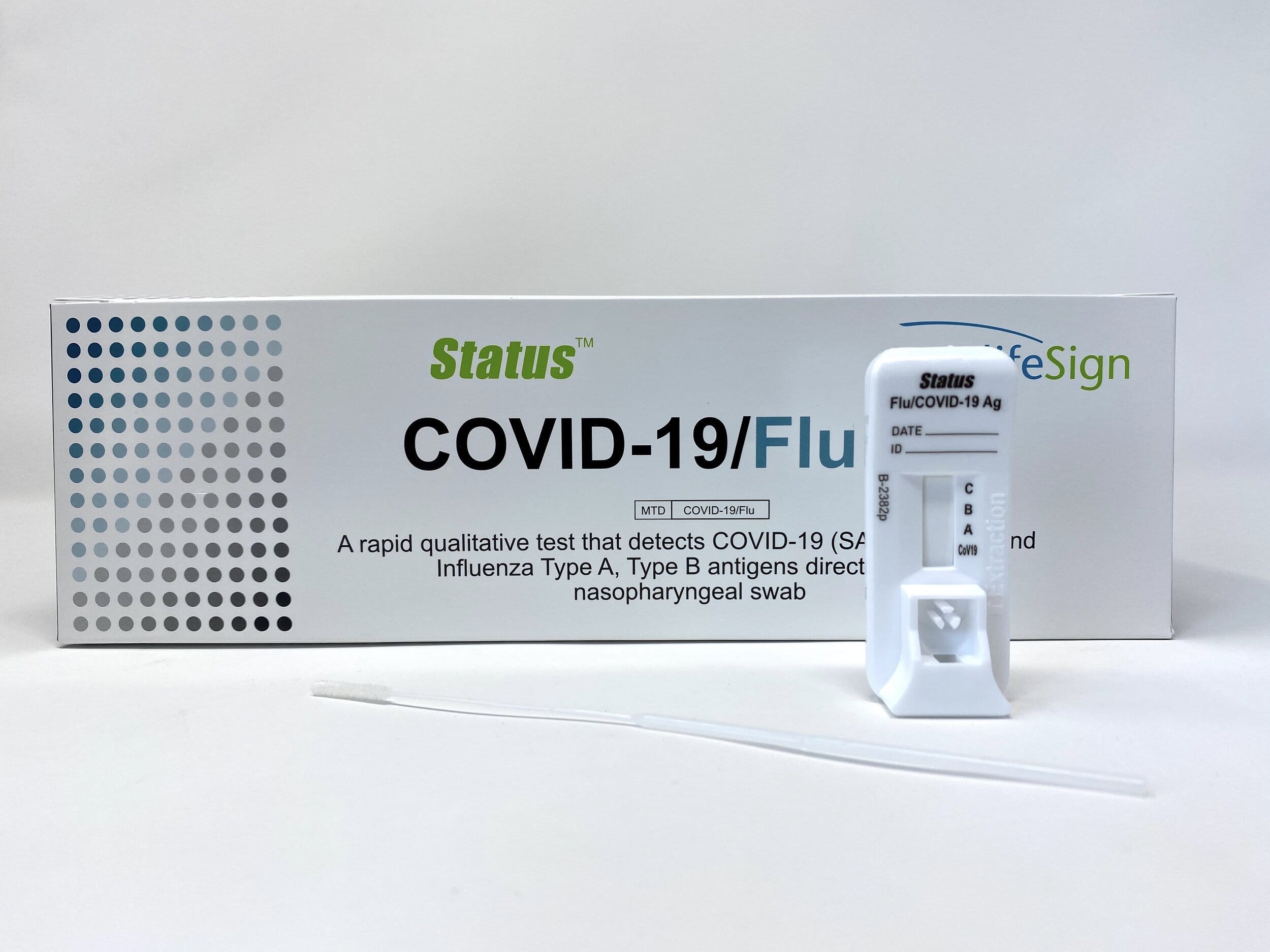
Status COVID-19/flu is a rapid, Point of Care immunochromatographic assay for the simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and influenza B directly from nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 within the first five days of onset of symptoms.
- Point-of-Care Rapid Test For 3 Viral Antigens From Just 1 Swab Sample
- Detects and Differentiates Between SARS-CoV-2, Influenza A and Influenza B Nucleocapsid Protein Antigen
- Broad Detection of Influenza A and Influenza B Strains, Including H1N1
- Superior performance for COVID-19: Sensitivity 93.9%, Specificity 100%
- Results in about 15 Minutes — Quick and Reliable
- Streamlined Procedure with Onboard Extraction — No Sample Transfer Required
- Pre-Measured, Unit Dose Reagent Capsule — No Counting Drops
- Easy-to-Read Visual Color Band Signal — No Reader Required
- Built-in Procedural Control
- Room Temperature Storage of Test Kit
Status COVID-19/flu is manufactured in the United States by Princeton Biomeditech Corp and was approved by Health Canada on September 24, 2021.
Buy Status COVID-19/flu for Your WorkPlace
If your company or organization is interested in rapid testing solutions, contact us today for help procuring Status COVID-19/flu or other rapid tests approved by Health Canada. Our team at Rapid Test & Trace Canada can also assist with implementing a rapid testing program to protect your workplace from the risks of COVID-19.









































