The ID NOW™ platform combines the benefits of speed and accuracy for the fastest molecular results in the market. The ID NOW™ COVID-19 test returns positive results in 13 minutes or less to enable immediate clinical decisions during the first patient visit. The easy to use ID NOW™ platform is designed for near-patient, point-of-care use in a variety of healthcare settings.
- Intuitive, CLIA-waived test procedure is designed for near-patient, point-of-care testing.
- Assay kit contains necessary testing components (24 tests, patient swabs and positive control swab) and can be stored at room temperature.
- Direct sample types include: nasal swab, nasopharyngeal swab and throat swabs
Id Now COVID-19 2.0 is manufactured in the United States by Abbott Diagnostics Scarborough, Inc. and was approved by Health Canada on April 8, 2022.
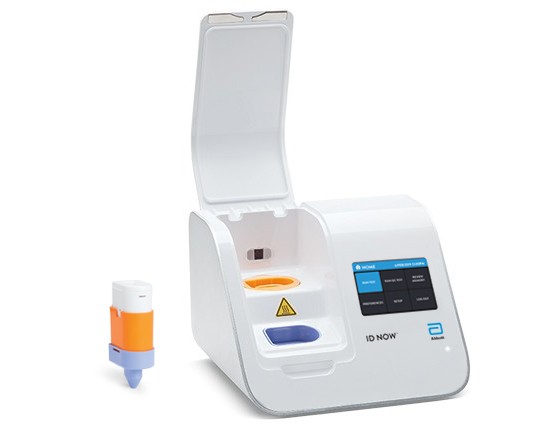
Buy Id Now COVID-19 2.0 for Your WorkPlace
If your company or organization is interested in rapid testing solutions, contact us today for help procuring Id Now COVID-19 2.0 or other rapid tests approved by Health Canada. Our team at Rapid Test & Trace Canada can also assist with implementing a rapid testing program to protect your workplace from the risks of COVID-19.












































