The Rapid Response™ antigen test device is the perfect screening tool for organizations that want to try to protect their workplace from the risk of COVID-19 lockdowns.
Used as part of a comprehensive rapid testing program for COVID-19, the BTNX nasal device offers fast, easy-to-use, and reliable screening. Testing all staff 1-2 times/week will help detect the virus early and decrease the likelihood of spreading COVID-19.
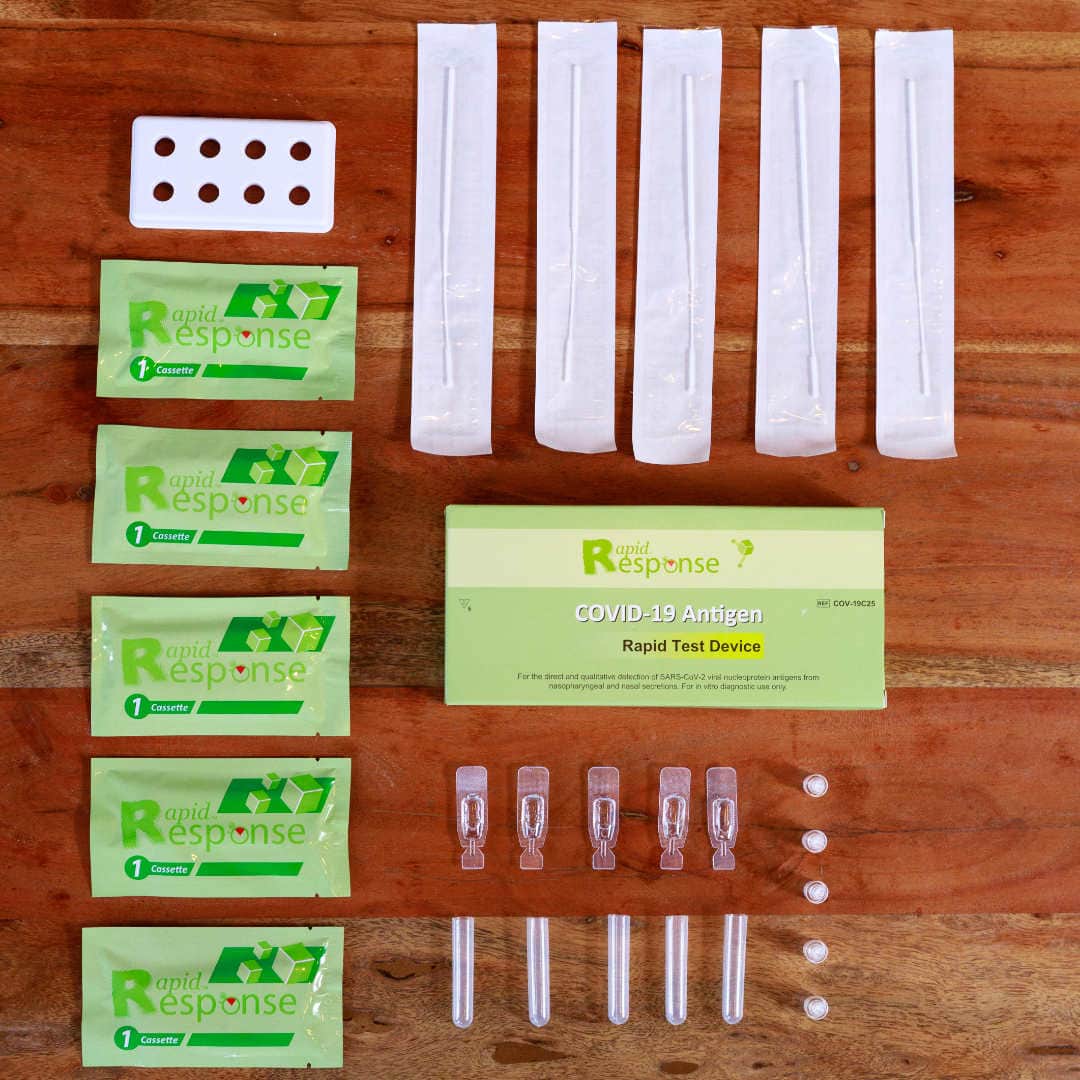
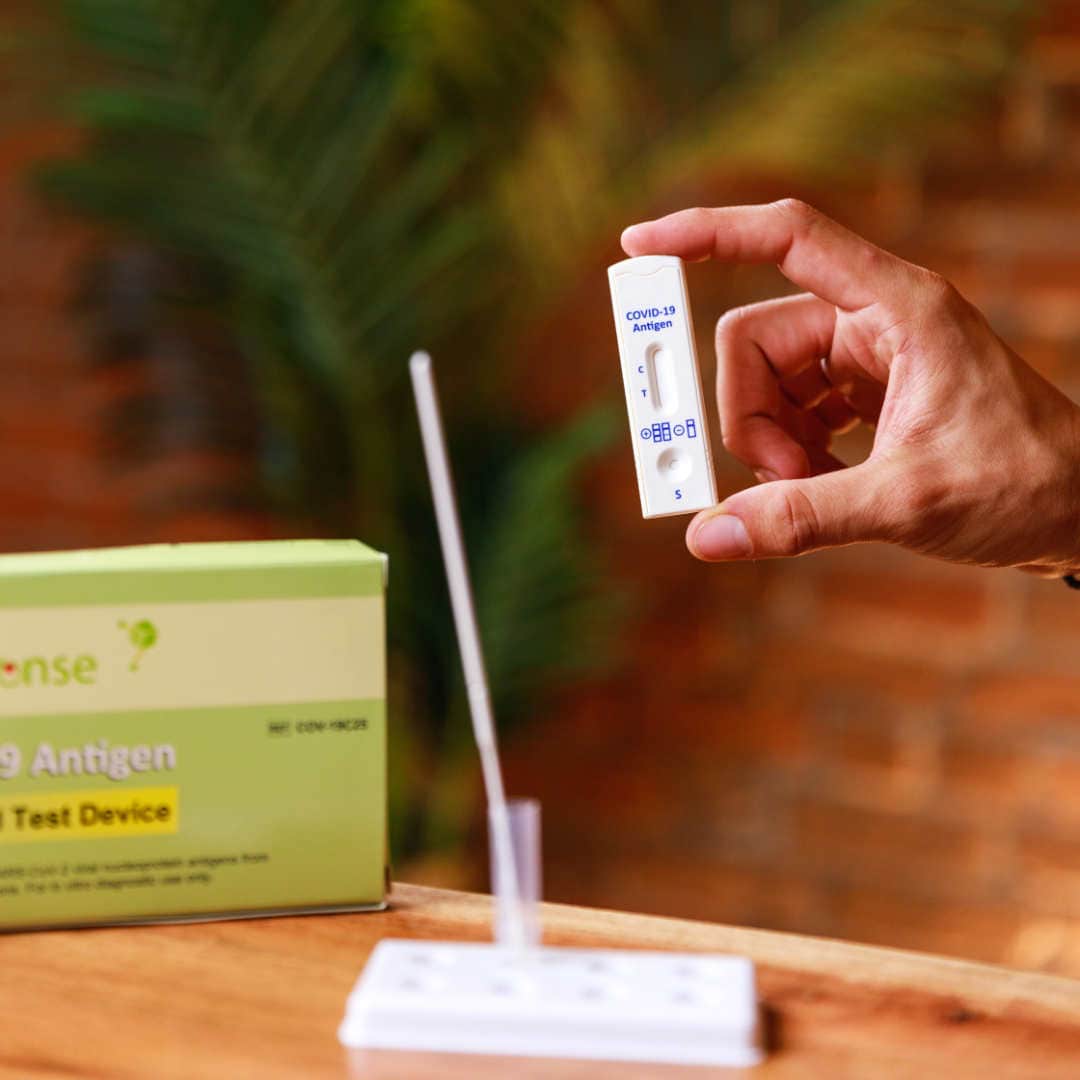
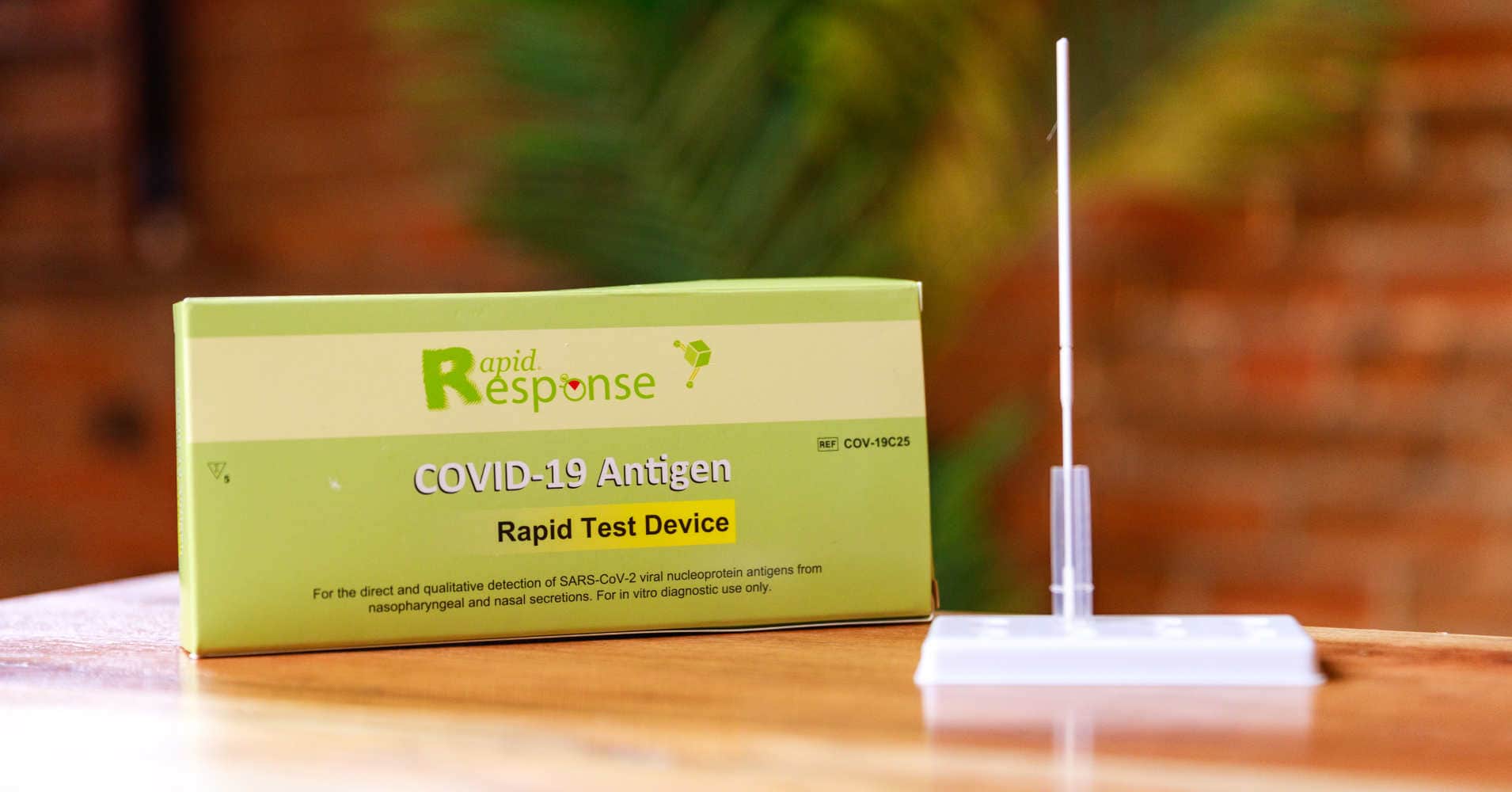
Rapid Response® COVID-19 Antigen Rapid Test Device. It is an efficient, accurate, and reliable method of testing that can be used by any healthcare professional. It is easy to use and delivers results within 15 minutes!
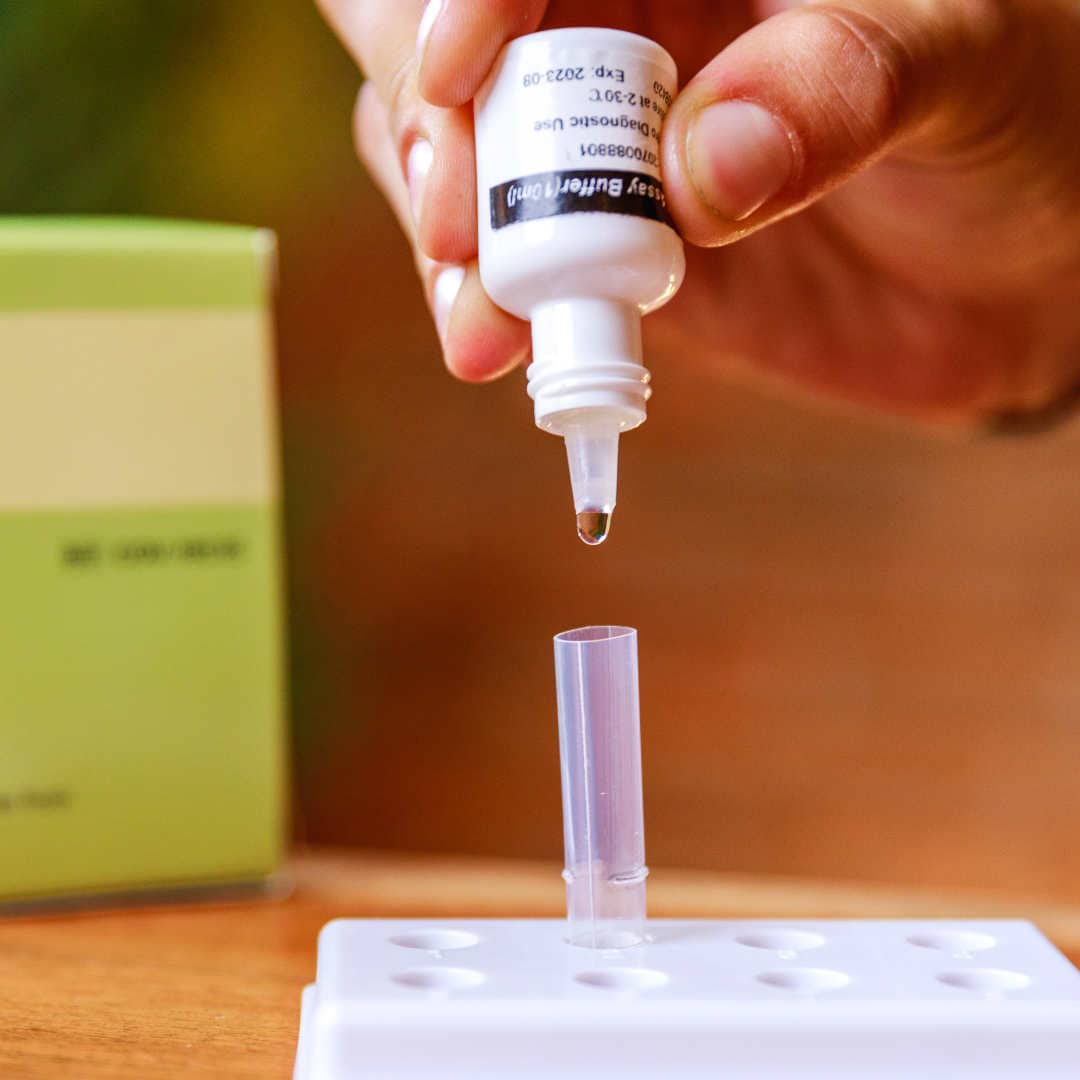
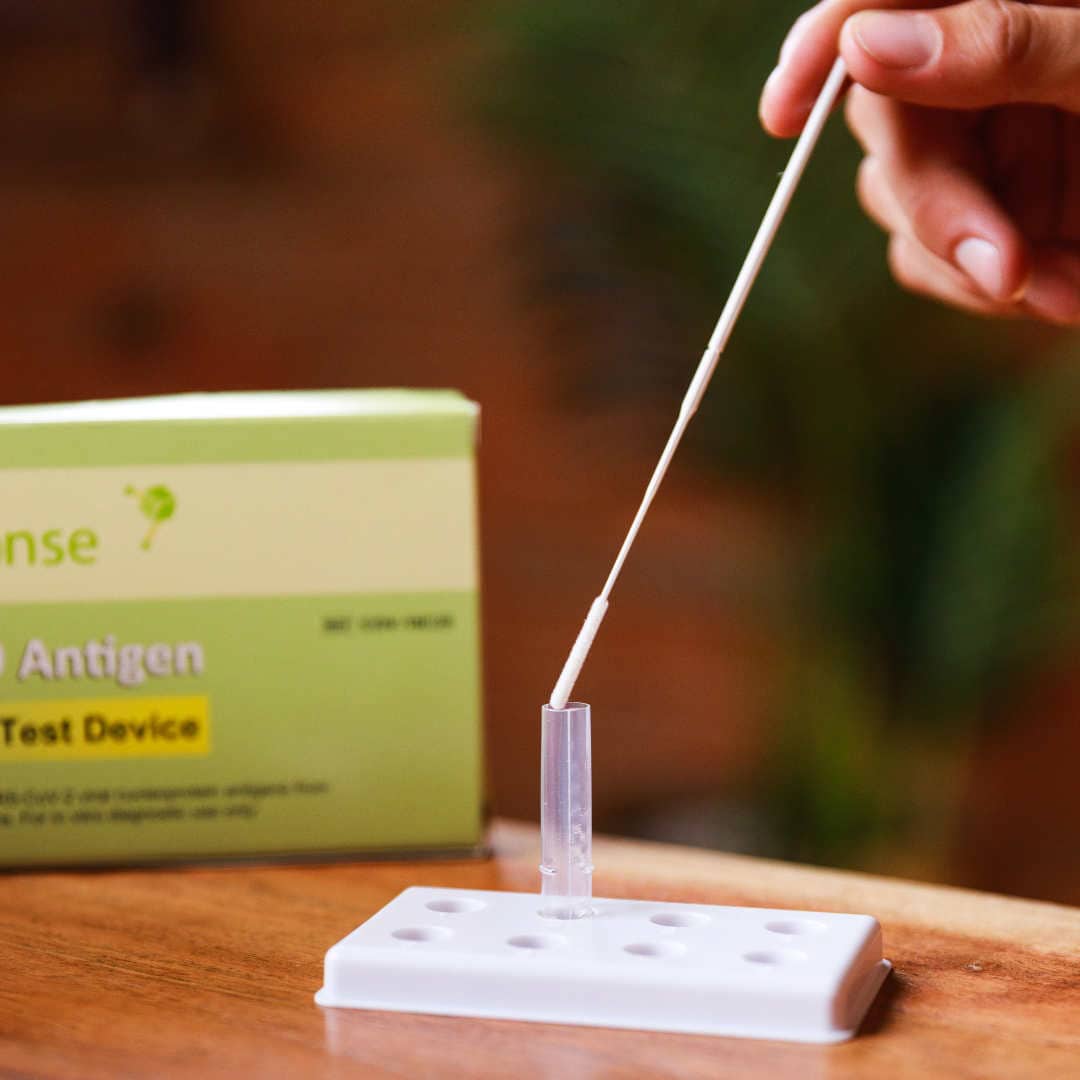
The BTNX Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal secretion samples.
Intended Use
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset. This test is authorized for use at the Point of
Care i.e., in patient care settings.
Results are for the identification of SARS-CoV-2 viral nucleoprotein antigen. Antigens are generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status.
Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories are required to report all positive results to the appropriate public health authorities.
Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient
management.
The Rapid Response™ COVID-19 Antigen Rapid Test Device is intended for use by trained laboratory personnel or health care professionals.
By purchasing this product, you agree to follow the manufacturer’s intended use and the sale conditions of this website.






















































Richard Park –
Our office uses the BTNX 25 pack for bi-weekly screenings. It’s quick, efficient, and offers clear results.
Tony Wilson (verified customer) –
The BTNX Rapid COVID-19 Test 25 pack is such a lifesaver. Great for quick screenings at home.
Paul Martinez (verified customer) –
I’ve tried a few rapid tests and the BTNX is by far the most reliable. Peace of mind in minutes.
Rachel West (verified customer) –
The convenience of having a 25 pack of the BTNX Rapid COVID-19 Test is unmatched. I feel prepared and safe.
Brooke Gonzales (verified customer) –
I was skeptical at first, but the BTNX Rapid Test proved its worth. Accurate and speedy results every time.