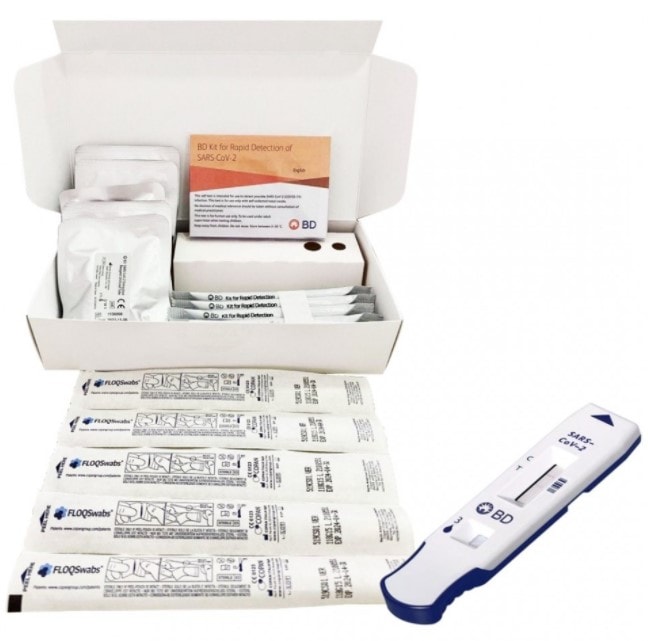
The BD Kit for Rapid Detection of SARS‑CoV‑2 is a chromatographic immunoassay intended for the direct and qualitative detection of SARS‑CoV‑2 nucleocapsid antigens in nasal swabs from individuals who are without symptoms, or with symptoms, who are suspected of SARS‑CoV‑2 infection.
- Assay results designed to be read visually
- Indicated for use in both asymptomatic and symptomatic individuals, who are suspected of SARS-CoV-2 infection by their healthcare provider
- Results in approximately 15 minutes
- Easily performed & interpreted by a healthcare professional or self-testing.
- Detection of SARS-CoV-2 antigen (nucleocapsid protein), in nasal swabs
- Reliable results, 91% PPA* compared to reference PCR method
- Provides intuitive sample processing with prefilled, single-use reaction tubes
The BD Kit for Rapid Detection of SARS-CoV-2 is manufactured in the United States by Becton Dickinson And Company and was approved by Health Canada on October 5, 2021.
Buy the BD Kit for Rapid Detection of SARS-CoV-2 for Your WorkPlace
If your company or organization is interested in rapid testing solutions, contact us today for help procuring the BD Kit for Rapid Detection of SARS-CoV-2 or other rapid tests approved by Health Canada. Our team at Rapid Test & Trace Canada can also assist with implementing a rapid testing program to protect your workplace from the risks of COVID-19.









































