Order Your 5 Pack of Rapid COVID Tests Today!
The 5 Pack of BTNX Rapid Response® antigen tests is the perfect screening tool for COVID-19.
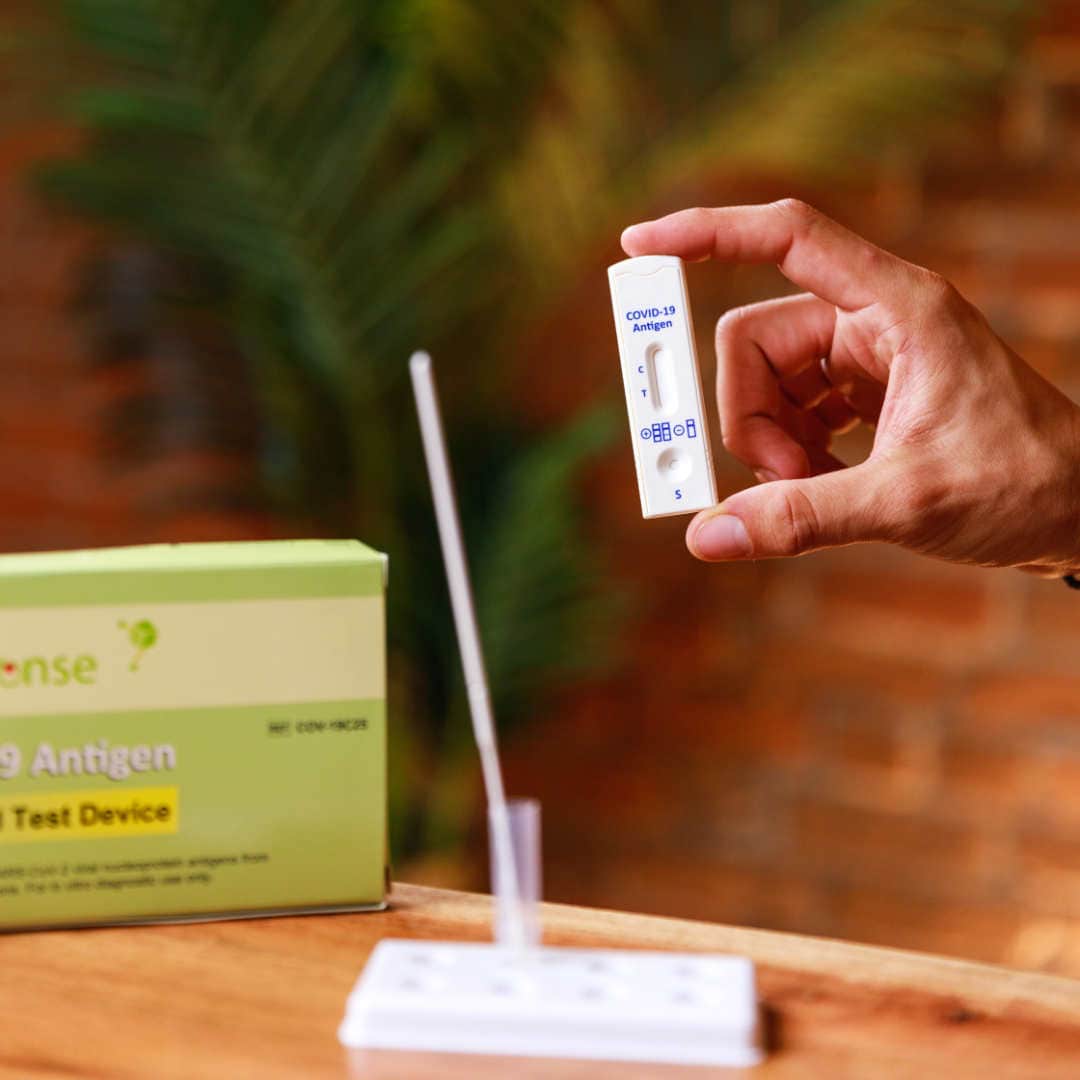
Put your mind at ease with a 5-pack of BTNX Rapid Response® COVID-19 Antigen Rapid Test Device. It is an efficient, accurate, and reliable method of testing. It is easy to use and delivers results within 15 minutes!
The BTNX Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal secretion samples.
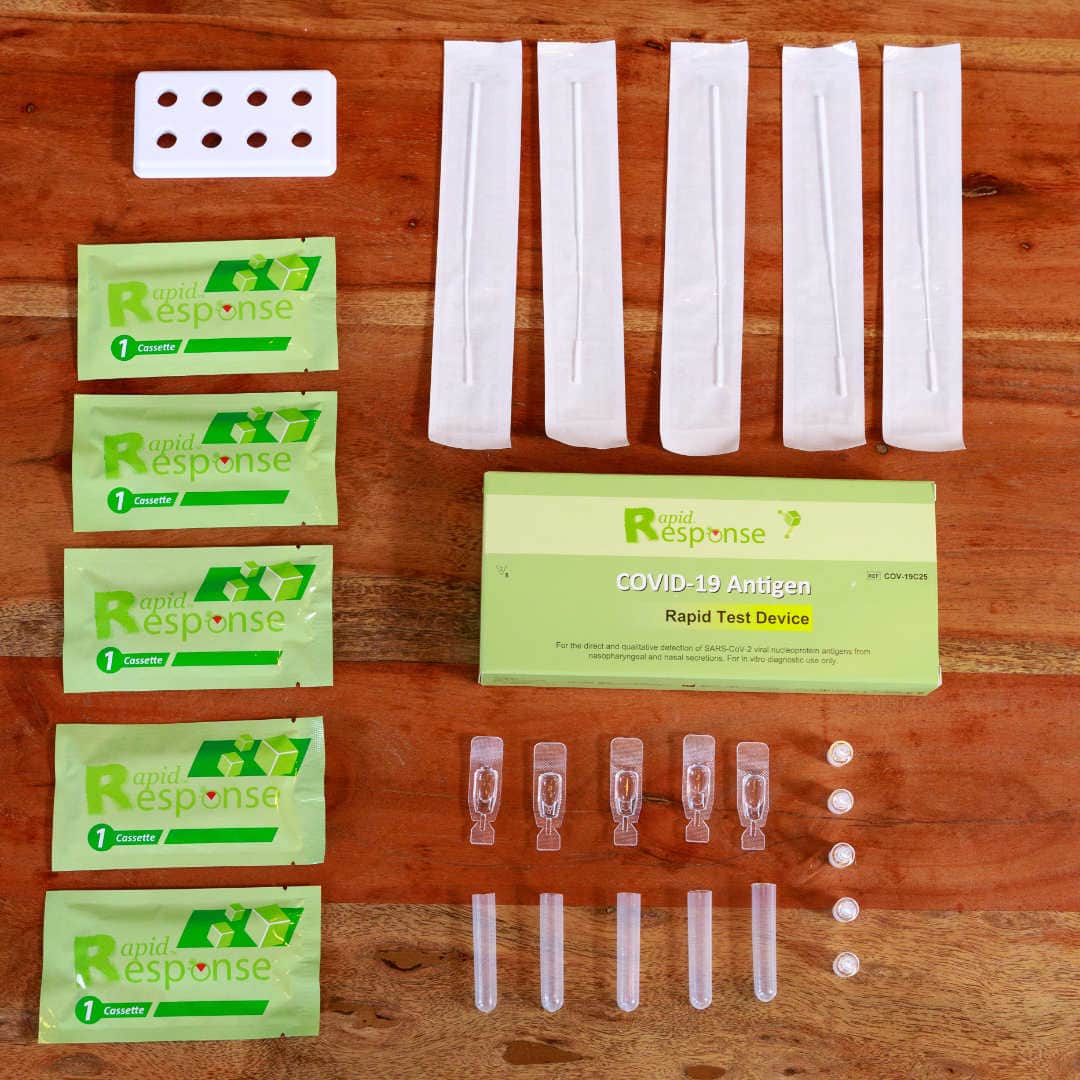
Package Contents:
- 5 Test cassettes
- 5 Individually packed swabs
- 5 individually packed buffers
- 5 tubes and nozzles
- 1 Tube stand
- 1 Product insert
Intended Use
The Rapid ResponseTM COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting.
Results are for the identification of SARS-CoV-2 viral nucleoprotein antigen. Antigens are generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories are required to report all positive results to the appropriate public health authorities.
Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with
a molecular assay, if necessary, for patient management.
The Rapid ResponseTM COVID-19 Antigen Rapid Test Device is intended for use by trained laboratory personnel or health care professionals.
By purchasing this product, you agree to follow the manufacturer’s intended use and the sale conditions of this website.






















































Jerry Patterson (verified customer) –
The BTNX 5 pack is a must-have for any household. Quick results and easy to administer.
Carmen Sherman (verified customer) –
Having the BTNX Rapid COVID-19 5 pack on hand has been such a relief. Traveling feels a bit safer now.
Sarah Taylor (verified customer) –
So glad I purchased the BTNX Rapid Test 5 pack. It’s a great deal for such a crucial product in these times.
Nancy Walker (verified customer) –
Bought the BTNX Rapid 5 pack for my family. Easy to use, and results are quick! Highly recommend.
Joseph Ramirez (verified customer) –
The clarity of instructions and efficiency of the BTNX Rapid COVID-19 5 pack is unparalleled. A great buy for sure.