The Lucira COVID-19 & Flu Test gives lab-quality results in the comfort of your home.
- 99% accurate compared to highly sensitive lab PCR tests
- Results in as fast as 11 minutes for a positive result and 30 minutes for a negative result
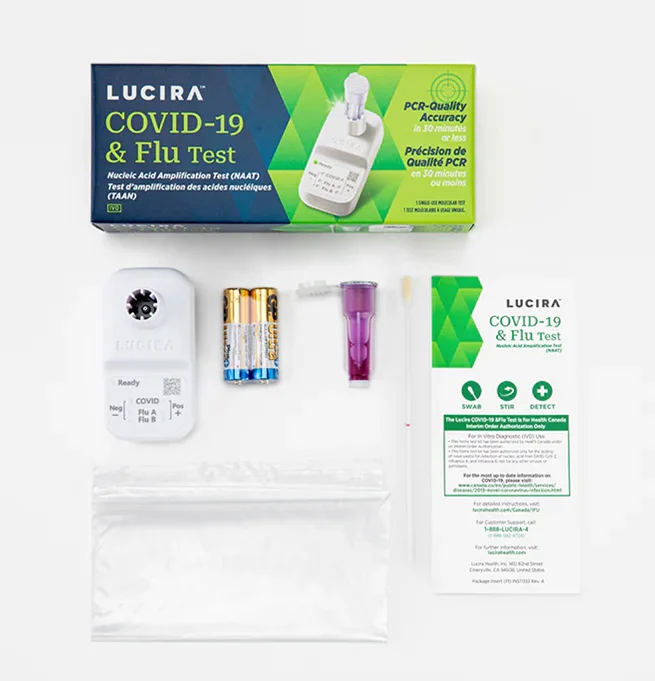
- One shallow nasal swab tests for Covid and all its variants of concern, Flu A and Flu B
- Health Canada authorized for ages 2+, with adult collection for children aged 2-13
- Extended expiry date: April 3, 2024
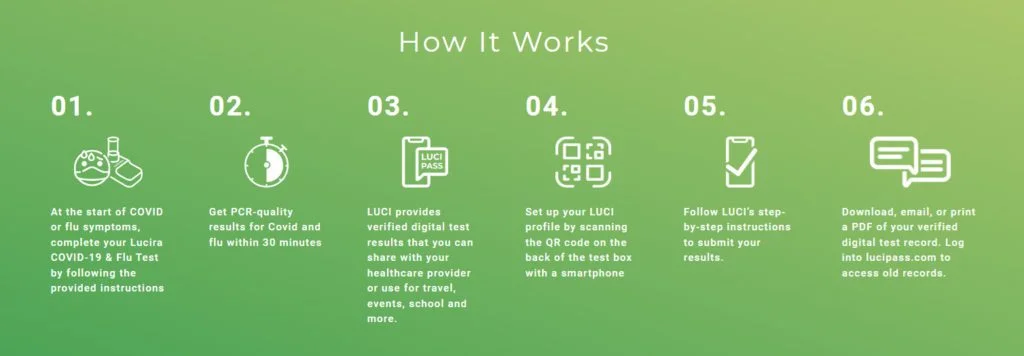
Instructions
The Lucira COVID-19 & Flu Test is a single use real-time RT-LAMP test intended for self testing at home for the simultaneous rapid qualitative detection and differentiation of RNA from SARS-CoV-2, Influenza A and Influenza B virus from self-collected nasal swab samples in individuals 14 years and older (self- collected) or individuals ≥2 years (collected by an adult) with symptoms of respiratory viral infection consistent with COVID-19, Influenza A and/or Influenza B. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar.
This test is also intended for use at the Point of Care (POC) for individuals with symptoms of respiratory viral infection consistent with COVID-19, Influenza A and/or Influenza B with specimens collected by trained personnel. The Lucira COVID-19 & Flu Test is intended for use as an aid in the differential diagnosis of SARS- CoV-2, Influenza A and Influenza B, in humans, and is not intended to detect Influenza C.
The SARS-CoV-2, Influenza A or Influenza B RNA is generally detectable in nasal swab samples during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, Influenza A or Influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Persons who test positive with the Lucira COVID-19 & Flu Test should seek follow up care with their physician, or healthcare provider, as additional testing and public health reporting may be necessary.
Negative results do not preclude infection from SARS-CoV-2, Influenza A, and/or Influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Intended Use
The Lucira COVID-19 & Flu Test is a single use real-time RT-LAMP test intended for self testing at home




















































Patricia Morrison (verified customer) –
Lucira’s rapid test combined with the prompt service from Rapid Test & Trace made for a stress-free testing experience. Reliable and efficient, I highly recommend.
Krista Jones (verified customer) –
The Lucira test was a life-saver before my recent trip. The quick turnaround time and straightforward procedure meant I could confidently travel knowing my status.
John Reynolds (verified customer) –
The speed of the Lucira test is commendable, but I’m concerned about its accuracy. I recommend following up with a PCR test for absolute certainty.
Kevin Wright (verified customer) –
The Lucira COVID-19 Test is a game-changer. It gave me results in under 30 minutes, right from the comfort of my home. I only wish the price was a tad lower, but for the convenience, it might be worth it.
Dell –
The Lucira COVID-19 Test made at-home testing a breeze. It was straightforward, with easy-to-follow instructions. Received my results in less than 30 minutes. Highly recommended for quick peace of mind!
Sally Meyer –
I was pleasantly surprised by how simple and painless the testing process was with the Lucira test. Clear instructions and got the results in minutes.
Michelle Smith (verified customer) –
It’s astounding how far we’ve come with medical tech. Lucira’s COVID-19 Test is evidence of that progress. It’s a user-friendly and efficient way to get tested, and I’ve found it to be consistently reliable.