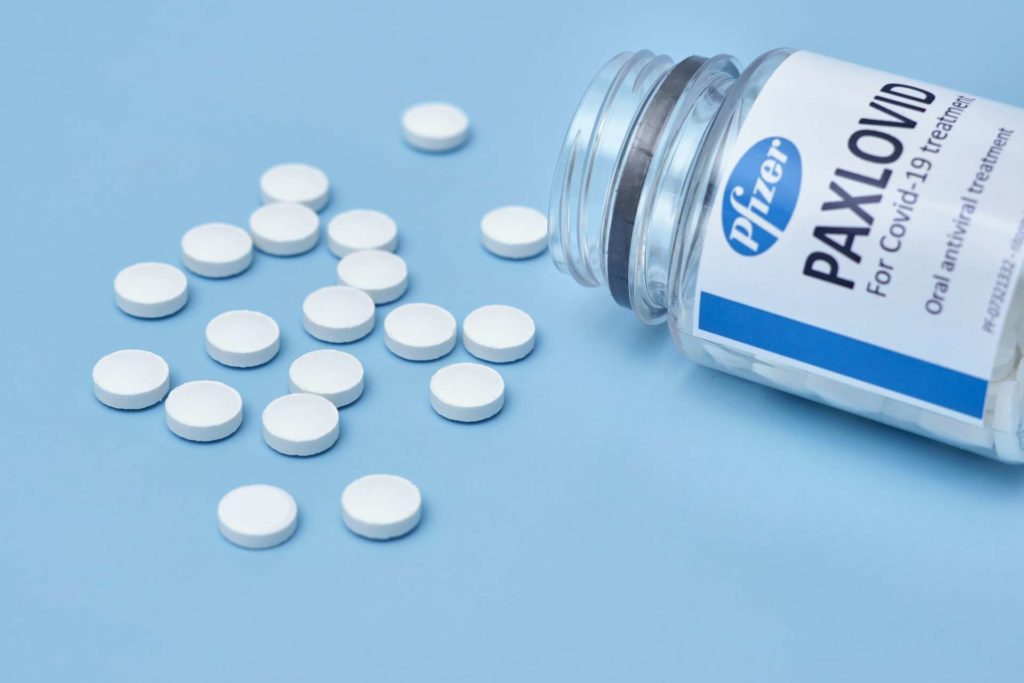
WHAT IS PAXLOVID AND HOW DOES IT WORK?
Paxlovid is an oral antiviral pill for COVID-19 patients who are exhibiting mild to moderate symptoms. It can be taken at home to help prevent high-risk patients from becoming severely ill to the point of hospitalization.
Paxlovid was approved by Health Canada on January 17, 2022. Prior to being authorized for use in Canada, it was granted an EUA (emergency use authorization) and received FDA approval in December 2021.
It has been classified as an investigational medicine, and is intended for adults and children (12 years of age and older) who meet the prescription criteria.
Paxlovid consists of two different antiviral medications, – nirmatrelvir and ritonavir.
They work in tandem to stop the COVID-19 virus from replicating, and in turn help to counteract the progression of symptoms in an individual who has been infected. More specifically, they are protease inhibitors.
Protease inhibitors have been used in a variety of industries to inhibit virus replication in the body by blocking the enzyme that facilitates the replication process.
Paxlovid’s antiviral components (protease inhibitors) effectively prevent the coronavirus from completing its lifecycle and continuing to spread.
HOW IS PAXLOVID USED?
Paxlovid is provided in pill form, and doses are taken orally. One dose consists of two nirmatrelvir tablets and one ritonavir tablet, co-administered twice daily for 5 consecutive days.
Antiviral medicines are most effective when taken as early as possible in the course of an illness. Paxlovid MUST be taken within five days of symptom onset.
- Take your second dose approximately 12 hours after. It is recommended you schedule your daily doses so they are easy to remember, for example, morning and evening.
- Take all three tablets (2 nirmatrelvir & 1 ritonavir) at the same time, or in immediate succession.
- Tablets should be taken whole – not chewed, broken or crushed.
- Be sure to complete the entire 5-day Paxlovid treatment regimen. Do not stop taking Paxlovid if your symptoms subside before the prescription has been finished as this can cause viral rebound and resistance to Paxlovid.
- Consult with your healthcare provider immediately if any issues arise.
PAXLOVID MEDICINAL INGREDIENTS
Pfizer’s Paxlovid consists of two medicinal ingredients: Nirmatrelvir and Ritonavir.
NIRMATRELVIR
Nirmatrelvir is a protease inhibitor that works by interfering with one of the key enzymes that the SARS CoV-2 virus needs to replicate and spread within the body.
Following a treatment of nirmatrelvir, the SARS-CoV-2 virus that is being released from infected cells will not be able to invade uninfected cells within the body – effectively interrupting viral replication and stopping the infection from spreading.
RITONAVIR
Ritonavir acts as a nirmatrelvir “booster” by helping to maintain the concentration of nirmatrelvir in the body. It does not have any direct effect on the COVID-19 virus.
Ritonavir is responsible for shutting down the liver’s metabolism of nirmatrelvir, allowing it to remain active and work to counteract the progression of the infection.
In other words, Paxlovid is essentially ritonavir-boosted nirmatrelvir.
DRUG INTERACTIONS WITH PAXLOVID
There are a number of other drugs that may actively interact with Paxlovid. Some of these drug interactions can result in adverse effects. Other medications that should be considered include:
- Drugs used to treat erectile dysfunction, such as tadalafil
- Drugs used to treat pulmonary arterial hypertension, such as bosentan
- Drugs used to lower blood cholesterol levels, such as atorvastatin and rosuvastatin
- Some drugs that are used to treat seasonal allergies and ear/eye infections, such as budesonide, fluticasone, prednisone and triamcinolone
- Some narcotic analgesics, such as fentanyl and tramadol
- Some blood thinners, seizure medications, hormonal birth controls & St. Johns wort
- Some sedatives or other drugs for treating anxiety, such as buspirone, diazepam, clorazepate and flurazepam
For a full list of drugs that may interact with Paxlovid visit https://covid-vaccine.canada.ca/info/paxlovid-en.html Inform your healthcare provider of any prescription medications, vitamins or herbal supplements that you are taking before moving forward with a Paxlovid prescription.
POSSIBLE PAXLOVID SIDE EFFECTS
Paxlovid is classified as an investigational medicine, which means that there are still some aspects possible that the risk remains unclear right now.
Paxlovid is known to be well-tolerated amongst most individuals.
However, possible side-effects may include:
- Increased blood pressure
- Muscle aches
- Abdominal pain
- Impaired sense of taste
- Diarrhea or indigestion
PAXLOVID & RENAL IMPAIRMENT
The Paxlovid oral antiviral is not recommended for individuals who have been diagnosed with severe renal impairment.
For people with moderate renal impairment, Pfizer has determined the appropriate dosage adjustment to ensure patient safety.
Individuals who are experiencing mild renal impairment do not need to have their Paxlovid dosage readjusted, and can follow the regular dosage guidelines.
IS PAXLOVID AVAILABLE TO EVERYONE?
Paxlovid has been authorized by Health Canada to treat adults and children above the age of 12 who are experiencing mild to moderate symptoms of COVID-19. Please note, here at Get Paxlovid we only see patients 18 years of age and over.
Taking Paxlovid is intended to help prevent high-risk patients from developing severe COVID-19 symptoms and progressing to the point of hospitalization.
Healthcare professionals have the ability to prescribe Paxlovid. It can also be provided (with some limitations) by a pharmacist. A full understanding of the patient’s medical history is necessary for determining appropriate dosage guidelines.
Patients must provide a positive COVID-19 test. Alongside a confirmed diagnosis, they must meet a number of other eligibility requirements in order to be considered for a Paxlovid oral antiviral prescription.
Women who are pregnant or breastfeeding should discuss their situation with a healthcare provider before taking Paxlovid.
To get your Paxlovid prescription through RT Medical’s convenient telehealth service, visit our calendar booking page.
It is important to remember that Paxlovid is still considered an investigational treatment. Consult with a healthcare provider if you have any other questions.
DOES PAXLOVID WORK AGAINST OMICRON?
The clinical trials conducted for Paxlovid began before the Omicron variant and other related subvariants became dominant.
However, Pfizer has stated that clinical data has proven that the drug is effective against Omicron and its subvariants.
There have been three laboratory-based studies performed that claim to have found scientific evidence that supports this claim. The studies were carried out by Pfizer themselves, as well as in conjunction with the Mount Sinai Icahn School of Medicine.
None of these studies have been published in peer-reviewed journals as of this point in time.
DOSAGE INFORMATION
One dose of Paxlovid consists of 3 oral antiviral tablets – 2 pink nirmatrelvir tablets and 1 white ritonavir tablet.
Dosage
300 mg of nirmatrelvir (2 x 150 mg tablets)
100 mg ritonavir (1 x 100 mg tablets)
Take all three tablets at the same time or consecutively, twice a day (in the morning and in the evening). Repeat this process for 5 days in a row.
Missed doses
If you miss taking your dose and it is:
Still within an 8 hour window of the time you would usually take your Paxlovid dose – take it as soon as possible.
More than 8 hours past the time you would usually take your Paxlovid dose – skip the missed dose and continue with the next dose at the normal time.
Overdose
If you suspect that you or someone else has taken too much Paxlovid, contact a healthcare professional, hospital emergency department or regional poison control center immediately.
IS PAXLOVID SIMILAR TO TAMIFLU?
Tamiflu is another antiviral drug that works by attacking the influenza virus to stop it from replicating and spreading through the body.
It works in the same way that Paxlovid does on the COVID-19 virus – effectively stopping the progression of symptoms while reducing the likelihood of severe illness, hospitalization or death.
A Tamiflu prescription is taken once daily for five days. It is that patients begin Tamiflu treatment within 48 hours of the onset of influenza symptoms.
Paxlovid and Tamiflu are antiviral drugs that are similar in function and are only deemed to be effective if taken within a certain time period after symptom onset.
WHAT OTHER TREATMENT OPTIONS ARE AVAILABLE?
In December of 2021 the FDA authorized the use of Paxlovid as well as one other similar oral antiviral treatment, known as Lagevrio. While Paxlovid has been approved, Canada has not yet approved the use of Lagevrio for COVID-19 patients.
Lagevrio (scientifically known as molnupiravir) is a nucleoside analog antiviral treatment that also works by stopping the COVID-19 virus from replicating. However, Lagevrio has a different method of accomplishing this in comparison to Paxlovid.
Molnupiravir imitates one of the key genetic components that the SARS-CoV-2 virus uses to replicate itself. When the medication is taken, the virus will insert molnupiravir into its genetic composition. This mistaken addition effectively stops the virus from replicating and continuing to spread infection.
Lagevrio is only meant for adults who are 18 years of age or older. One dose consists of 4 molnupiravir tablets taken twice daily for 5 consecutive days.
Clinical trials conducted for Lagevrio showed a decrease in the risk of COVID-19 hospitalization or death by approximately 30 percent in high risk patients.
Paxlovid, on the other hand, has been shown to be over 80 percent effective in preventing the development of severe symptoms. The FDA recommends the use of Paxlovid instead of Lagevrio if available.
ARE COVID-19 VACCINATIONS STILL NECESSARY WITH PAXLOVID?
Paxlovid is not a substitute for vaccination against COVID-19. It is meant to treat patients who have already been infected with the virus, are dealing with severe disease or illness, and are at risk for hospitalization due to symptom progression.
COVID-19 vaccines are intended to lower the risk of becoming infected and then spreading the virus to other healthy individuals.
Paxlovid should be taken in response to infection with the COVID-19 virus. It is NOT a substitute for vaccination.
FAQs About Pfizer’s Paxlovid in Canada
What is Paxlovid COVID-19 rebound?
Paxlovid COVID-19 rebound is a term that refers to the experience of returning (rebounding) virus symptoms and/or another positive COVID-19 test after having completed a Paxlovid treatment.
Experts have said that the rebound of COVID-19 symptoms following a Paxlovid treatment is most likely due to insufficient drug exposure – an insufficient amount of the drug got into infected cells to completely stop viral replication.
This may be due in part to the active medicinal ingredient being metabolized by the body more quickly in some individuals than others.
Can you get re-infected with COVID-19?
Yes, it is possible to get re-infected with the COVID-19 virus after an initial infection and recovery.
Before the arrival of the Omicron variant, individuals who had already been infected with COVID-19 were at an 84% lower risk of re-infection.
The emergence of Omicron and its subvariants showed high transmissibility and an increased resistance to immunity. Since then, the number of re-infection cases reported has increased substantially.
Even if an individual was infected with the original Omicron variant, newer subvariants of Omicron (for example BA.5) can cause re-infection regardless.
How well does Paxlovid work?
Pfizer conducted a study that involved a number of high-risk, unvaccinated adults over the age of 65 and/or whom have underlying medical conditions.
The study showed that treatment with Paxlovid was able to decrease the likelihood of severe illness, hospitalization or death by approximately 89%.
Paxlovid is one of the most effective oral antivirals that is currently available for counteracting the effects of infection with COVID-19.
What is the effectiveness of Paxlovid in children?
Paxlovid clinical trials and other laboratory-based studies have not been conducted for children with COVID-19. It is not currently known if children will experience the same benefits or different side effects as adults when treated with Paxlovid.
Do I need to take Paxlovid if I am not feeling sick?
Paxlovid is intended to be used by an individual with COVID-19 as early as possible in the infections progression. Paxlovid must be taken within 5 days of symptoms arising in order to be fully effective.
It is possible that some individuals infected with COVID-19 will not necessarily feel sick. This does not mean that you do not need to take Paxlovid if you are someone who is at risk for the development of severe illness.
Ultimately, the decision is up to the individual themselves.
What is an Emergency Use Authorization?
The United States FDA has approved the distribution of Paxlovid through an Emergency Use Authorization (EUA).
EUA authority allows the FDA to help the nation’s public health organizations to protect people against chemical, biological, radiological and nuclear threats.
These threats include infectious diseases, which in some cases merit the approval and use of medical countermeasures necessary in the event of public health emergencies.